G Dranoff Difference Between Innate and Adaptive Immunity Nature Reviews
Introduction
From the beginning, cancer handling approaches with chemotherapy and radiation therapy have largely been unable to discriminate between good for you and cancerous cells in their killing action. These therapeutics therefore often sacrifice healthy tissue in the try to treat the cancer, which too often results in temporary, merely often harsh side effects, and long-term consequences, including secondary malignancy. In add-on, these treatment methods neglect to account for cellular heterogeneity between individual patients with the aforementioned diagnosis, or even within a unmarried tumor itself. Although withal controversial, evidence indicates that in certain cancer types, pocket-sized portions of cancer cells situated within an individual tumor display "stalk-cell" like properties and are resistant to such therapy, such that they proceed to proliferate in spite of treatment (3).
Cancer immunotherapy seeks to strengthen and direct the patient's natural immune mechanisms against cancerous cells, with the aim of targeting the affliction while minimizing effects to surrounding healthy tissue (1). Preliminary information brandish the potential of immunotherapy, specifically natural killer (NK) jail cell-based immunotherapy, for targeting the quiescent cancer stalk cell (CSC) population (3). Although such approaches have been discussed for decades, just recent advances in our agreement of cancer immunology accept immune for direct applications regarding these more specialized therapies (2).
What is in a Tumor: That Which I Call Heterogeneity
"Tumor heterogeneity" refers to differences betwixt tumors of the same type in different patients, every bit well as to differences among cancer cells within the same tumor. Both can atomic number 82 to various responses to therapy and may explicate why some tumor cells remain post-obit aggressive treatment (4). There are two predominant theories to explain tumor heterogeneity, the CSC, or hierarchical model, alluded to above, and the stochastic model. The CSC model argues that individual tumors are comprised of a combination of both tumorigenic and non-tumorigenic cells, which are derived from a single CSC that undergoes transformation as a effect of both genetic and epigenetic influences. Theory holds that the vast bulk of the cells in these cancers take picayune capacity to contribute to disease progression, thus information technology is necessary to focus therapy on the small subpopulations of tumorigenic cells rather than the tumor as a whole (5). By contrast, in the stochastic model, all tumor cells are considered biologically equivalent simply vary in behavior and office based upon intrinsic and extrinsic influences (6). Although at that place is compelling show to support the CSC theory in many types of cancer, including leukemias, breast cancers, encephalon cancers, and colon cancers, fence continues as to whether this model tin can be applied widely to all cancers, or whether certain cancer types showroom the stochastic model (5).
Muster Your Wits and Stand in Your Own Defense: The Immune Organization
The human immune organization is divided into two arms, innate and adaptive, which work cohesively in response to specific internal and external stimuli. The innate allowed system is involved in immediate host defense force to perceived pathogens and includes neutrophils, monocytes, macrophages, complement, cytokines, and acute phase proteins. Adaptive immunity consists of antigen-specific reactions mediated past T lymphocytes and B lymphocytes.
Natural killer cells are lymphocyte-similar cells considered to exist role of the innate immune system, which recognize and answer to abnormal cells—typically either virus-infected or malignant cells—in two means. First, they demark antibody coated targets via immunoglobulin receptors leading to antibody-dependent cellular cytotoxicity (ADCC). 2nd, they bear natural cytotoxicity receptors (NCRs) that discover the altered expression of ligands on the surface of tumor cells, which ultimately triggers NK cell activation. These mechanisms are illustrated in Figures 1A,B. NCRs are also involved in the procedure of discriminating betwixt cocky and non-self via the generically termed MHC I receptor and ligand (7, viii).

Effigy 1. Natural killer (NK) cell interaction with cancer prison cell microenvironment. (A) Antibiotic-dependent cellular cytotoxicity. The NK cell Fc receptor, CD16, binds the Fc region of the IgG antibody bound to the tumor antigen, leading to NK cell protease release and subsequent tumor cell lysis. (B) NK cell activation via natural cytotoxicity receptors (NCRs). The NCR KIR, an inhibitory receptor, recognizes the absence of MHC I ligand on the surface of the cancer cell. Because the inhibitory receptor remains unbound by MHC I, inhibition does not occur, and the NK jail cell is thus activated, leading to tumor cell lysis. Note: the NK cell killing mechanism proceeds principally by proteolytic lysis. For greater detail on mechanisms of cancer cell killing, see Ref. (9).
Will Yous Yield and This Avert: Potentiation of NK Cells
Every bit mentioned higher up, NK cells are widely circulating lymphoid cells, specialized to eliminate virus-infected and malignant cells. Although traditionally categorized under the umbrella term of the innate immune system, contempo evidence has indicated that NK cells tin can develop prolonged and highly specific memory to various antigens, a property typically associated with the adaptive allowed response (ten).
Studies have indicated that NK cells are frequently deficient or dysfunctional in patients with malignancy, indicating that this may exist a central factor in cancer immunoevasion and progression. In a follow-up study of a patient cohort examining natural cytotoxic activeness and cancer incidence, low NK prison cell part was found to predict an increased risk of developing cancer, thus further supporting their role in malignancy attenuation (11, 12). Given our expanding knowledge of routine NK prison cell function in conjunction with show of consequences of their dysfunction under the circumstances of malignancy, information technology is reasonable to conclude that the next advancement in cancer therapy should involve NK jail cell regulation and adaptation. Several approaches for NK prison cell utilization have been published, and many more are currently being evaluated. Such is the bailiwick for the residual of this study.
Become Forth and Multiply!: NK Cell Population Enhancement
For decades, allogeneic hematopoietic prison cell transplantation (HCT)—the engraftment of a donor's immune system into a recipient with the objective of eliminating cancer cells—has provided increased disease-costless survival to patients with hematologic malignancies. However, such methods come up with the run a risk of graft versus host illness (GVHD), a life-threatening condition wherein recipient cells are recognized equally foreign and attacked by the donor immune cells. One potential alternative to this is to isolate specific donor antitumor cells, most notably NK cells, for use in HCT, which minimizes the risk of GVHD. Such methods have displayed positive clinical outcomes in astute myelogenous leukemia (AML) patients who underwent HLA-haploidentical NK cell-specific HCT (11, 13).
Natural killer cells can be obtained from either patients themselves (autologous) or from a donor (allogeneic) and can be derived from multiple sources, including peripheral blood, bone marrow, or umbilical string blood. Under normal circumstances, few NK cells circulate in man claret, and those that practice frequently showroom express cytotoxic activity due to immaturity (xiv). In addition, NK cells are brusk lived, with an average life span of 2 weeks (15). Thus, research has focused on developing methods to expand NK prison cell populations, increment their life span, and potentiate their cytotoxicity. Preliminary studies displayed promise with in vitro expansion of NK cells using the cytokine IL-2. Nevertheless, do good is limited in vivo due to the high affinity of T-regulatory cells for IL-2, which is responsible for NK jail cell inhibition to forbid autoimmunity, besides as by the short half-life—approximately 10 min—of IL-ii in serum (8, 14). Contempo testify has indicated that the inhibition of NK cells can be overcome by diminishing the T-regulatory jail cell population through the apply of an IL-2-diphtheria toxin fusion poly peptide (IL2DT) (16, 17). IL2DT, too known as denileukin difitox or Ontak, is a recombinant cytotoxic fusion protein comprised of IL-two and diphtheria toxin. The toxin selectively depletes IL-ii-receptor expressing cells, specially those bearing the IL-2 receptor α chain isoform, such as T-regulatory cells. IL2DT has a brusk half-life, thus donor NK cells infused hours later would be unaffected by it, and in fact, would proliferate more than due to the transient decrease in their suppression by T-regulatory cells. A stage II clinical trial from the University of Minnesota that examined 57 patients with refractory AML displayed improvements in rates of NK-prison cell expansion and AML remission in 27 and 53% of patients, respectively, in those who received IL2DT as compared to 10 and 21%, respectively, in those who did non receive it (16). Other information indicated that modifying NK cells to produce IL-two prior to transplantation can be done to create a cocky-sustaining source of IL-2 in vivo, providing additional possibilities for combination therapies (8).
Besides solitary IL-2, the combination of the cytokines IL-18, IL-fifteen, and IL-12 has shown promise in inducing the proliferation of retentivity-like NK cells in immunodeficient mice when accompanied past supplemental exogenous IL-2 (fourteen). Purified man NK cells cultured overnight in IL-2, IL-12, IL-15, and IL-eighteen that were afterwards infused into mice displayed increased responsiveness to exogenous IL-ii; this was thought to exist due to the consecration of increased CD25 expression on the NK cells, a cardinal component in the germination of the high-affinity IL-2 receptor subtype, IL-2Rαβγ (18). Many other combinations, including both cytokines—most recently IL-27—and other substances such as intravenous immunoglobulin, accept been or are currently undergoing evaluation for their prospective roles in NK cell population modulation (14, 19, twenty).
Oh, Sweet Intoxication: Disinhibition of NK Cells
Like true lymphocytes, NK cells have a number of endogenous mechanisms that balance cocky-defense with self-recognition. First, they express MHC I receptors which interact with different cell types in the surrounding surround. If the MHC I receptors remain unbound upon interaction with a particular prison cell, that cell is targeted for lysis by the NK cell. Second, they express activating receptors which recognize stress-induced ligands on the surface of target cells and trigger prison cell lysis upon detection. Finally, they limited the activating receptor CD16 that facilitates ADCC upon binding the Fc portion of various IgG antibody isotypes. In addition, NK cell action is too modulated by diverse cytokines, toll-similar receptor ligands, and T-regulatory cells (vii, 14). To optimize NK cell antitumor responses, preliminary studies have examined approaches to cake NK prison cell inhibition.
Show has shown that the antigen-specific NK prison cell targeting machinery via CD16 plays a vital role in the effectiveness of established tumor specific monoclonal antibody (mAb) therapies such as trastuzumab, rituximab, and elotuzumab due to CD16 interaction with the Fc portion of the mAb that coats the tumor cells, as illustrated in Figure 2A (21). However, several studies accept noted the downregulation of CD16 on the NK cells of cancer patients in comparison to salubrious patients, making mAb therapies less effective due to decreased ADCC (22, 23). Thus, a developing approach to enhance NK cell-mediated ADCC responsiveness to tumor cells is to inhibit the shedding of CD16 via the inhibition of metalloproteinases (MMP) that cleave them, every bit shown in Figure 2B (21). Furthermore, genotypic variations be in CD16 between individuals, which tin can influence its interaction with immunoglobulins; this not only results in variations in mAb therapy effectiveness depending on CD16 genotype but as well affords a potential opportunity for targeted therapy (24). Consequently, a multifactorial approach bookkeeping for all of these elements could greatly increase NK cell responses to malignancy.
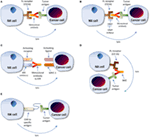
Effigy 2. Modulation of natural killer (NK) cell interaction with cancer cell microenvironment. (A) Monoclonal antibody (mAb)-driven cellular cytotoxicity. mAbs specific to the tumor antigen demark the tumor jail cell. The NK cell Fc receptor, CD16, binds the Fc portion of the mAb bound to the tumor cell, leading to NK prison cell activation, protease release, and tumor cell lysis. (B) Metalloproteinase inhibition combined with mAb therapy. Metalloproteinase inhibitors preclude cleavage and subsequent shedding of CD16 receptors by blocking MMP action. In conjunction with this enhancement in CD16 receptors, mAbs specific to chosen tumor antigen enhance NK cell binding via CD16 receptor, leading to NK jail cell protease release and tumor cell lysis. (C) mAb to KIR. The Fab region of an mAb specific to KIR binds the receptor. The antibody blocks KIR from bounden the tumor cell MHC I ligand, thus preventing cellular inhibition, consequently leading to NK prison cell-mediated tumor prison cell lysis. (D) BiKEs and TriKEs. The Fab region of an antibody is designed to target both the Fc receptor of the NK prison cell also as at least one specific tumor antigen, thus leading to NK cell stimulation and tumor cell lysis. (Eastward) Chimeric antigen receptors (Machine). A CAR specific to a called tumor antigen is designed and infused into the NK cell, thus enhancing the NK cells' recognition of the tumor cell. The NK cell and then increasingly targets tumor cells for destruction. Annotation: the NK prison cell killing machinery gain principally past proteolytic lysis. For greater detail on mechanisms of cancer cell killing, run into Ref. (ix).
One such example of these multifactorial strategies in practise is the use of GM6001, an MMP inhibitor used for in vitro treatment of NK cells. NK cells treated with GM6001 displayed preserved CD16 expression despite initial losses stimulated by the presence of tumor cells (25). Further studies accept confirmed that a specific metalloproteinase, ADAM17, is able to mediate CD16 shedding in NK cells thus providing another promising opportunity for targeted cancer immunotherapy. A preclinical trial that examined the combination of an MMP inhibitor and anti-CTLA4 antibodies in mice with breast cancer displayed delayed tumor growth and metastases reductions in mice treated with MMP inhibitor verses those treated with anti-CTLA4 antibody lone. Anti-CTLA4 antibodies are involved in inhibiting allowed system downregulation (26). A phase I/II clinical trial examining an MMP inhibitor which specifically targeted ADAM17 and the like ADAM10 in combination with trastuzumab in patients with HER2+ metastatic chest cancer displayed no meaning improvement from controls (27). While not specific to NK cells, nonetheless these results indicate a necessity for further exploration of this potential therapeutic method.
Another possible, albeit distinctive approach, is the use of an mAb, IPH2101, which blocks KIRs, preventing their binding to MHC I ligands, and thus perpetuating NK jail cell-induced target prison cell lysis, equally illustrated in Effigy 2C. Although initial results using the agent alone were inconclusive, a more recent study combining information technology with lenalidomide, an anti-angiogenic agent, in patients with multiple myeloma was more than promising (11).
Alternative therapies maximizing the activating receptors expressed by NK cells have also been developed. NKG2D is a lectin-like activating receptor expressed past NK cells, activated CD8+ T cells, and macrophages, which reacts to NKG2D ligands found most commonly on tumor cells. Expression of these NKG2D ligands by target cells triggers NK cell cytotoxicity and target cell lysis (28). This mechanism was recently utilized in a study examining the suppression of colon cancer in mice. In the study fluorescent-labeled gene nanoparticles consisting of gene fragments of IL-21, a cytokine involved in lymphocyte activation and tumor suppression, and NKG2D were developed and intravenously injected into mice pretransplanted with tumors. The dsNKG2D-IL-21 nanoparticles preferentially amassed in the tumor cells which could after continue to secrete the dsNKG2D-IL-21 poly peptide, farther activating T and NK cells against the tumor tissue. Although further investigation is required, the antitumor furnishings displayed by such strategies are promising (29).
Worthy of Recognition: Enhancing Tumor Antigen Recognition
Due to the evidence that NK cells harbor allowed retentivity and antigen recognition properties, therapeutic approaches take at present evolved to exploit them. Initial studies utilized KIR mismatch between donors and recipients to enhance donor prison cell recognition of recipient leukemia, which was found to improve non-relapse bloodshed and overall survival (11, 30). A prime example of this mechanism in action was shown in an influential study completed by kinesthesia at the University of Perugia and Stanford University, in which 60 high-risk leukemia patients received hematopoietic cell transplants from family donors mismatched by both HLA haplotypes and KIR epitopes. NK cells that were initially shown to lyse allogeneic B-cell lymphoblastoid cells and PHA lymphoblasts in a subset of the patients (4 CML, 4 AML, 5 ALL) were isolated and used to target pretransplant cryopreserved leukemic cells from the same 13 patients. The isolated alloreactive NK cells killed all of the acute and chronic myeloid leukemia samples, and two of v acute lymphoblastic leukemia samples (31).
Knowledge of NK jail cell triggering via CD16 past mAb therapies as discussed above has led to the development of bi- and trispecific antibodies—frequently referred to as bi- and trispecific specific killer prison cell engagers (BiKEs and TriKEs)—which enhance the specificity between NK cell and tumor. This method involves designing bi- and trispecific antibodies that fuse the Fab region of the antibody to the specific tumor jail cell antigen in combination with another Fab region of the aforementioned antibody fused with the CD16 portion of NK cells, which leads to NK prison cell stimulation and tumor cell lysis. Multiple Fab regions can be utilized to specifically target multiple tumor antigens (viii). This mechanism is illustrated in Figure 2nd. The efficacy of this method was displayed in an in vitro written report done past the University of Minnesota that combined an MMP inhibitor to ADAM17 with a Wheel targeting CD33 through CD16 in the treatment of refractory AML cells. The combination therapy was plant to enhance NK cell cytotoxicity and cytokine release against the CD33+ malignant cells (32).
Another method of enhancing tumor antigen recognition involves the use of single chain fusion proteins—referred to as chimeric antigen receptors (Auto)—molecules artificially engineered and introduced into hematopoietic cells such as NK cells or T lymphocytes to redirect specificity toward a chosen antigen, as shown in Effigy 2E (eight, 33). Automobile modifications of T cells have been researched extensively; however, evaluation of this possibility in NK cells remains in its infancy (8). One recent study which showed much hope involved the transduction of man NK cells with a Auto targeting both wild-type and mutant epidermal growth cistron receptors (EGFRs) commonly expressed by glioblastoma (GB) cells. The EGFR-Motorcar-engineered NK cells displayed increased interferon-γ production and increased tumor cell cytotoxicity when cocultured with GB cells, likewise equally increased malignant jail cell growth suppression when administered intracranially in mice (34). Other preclinical studies have successfully transduced human NK cells to express CARs to CD19, CD20, CD244, and HER2, which all displayed efficacy in tumor jail cell lysis (35). A phase I clinical trial by St. Jude'due south in 14 relapsing or refractory B-lymphocyte astute lymphoblastic leukemia patients involving the transduction of NK cells to express a CD19 CAR concluded in February 2015 with no published results to this appointment (8, 36, 37). A second phase 2 pilot study also involving the engineering of an anti-CD19 CAR at the National University Health Organization in Singapore is currently recruiting participants (8, 36, 37).
Plan for the Time to come considering That is Where Y'all are Going to Spend the Rest of Your Life
Every bit discussed above, mounting evidence indicates that many tumors comprise CSCs, rendering them incomparably resistant to established treatments. Recent reports have indicated that CSCs may be preferentially susceptible to NK cell killing, due in part to their decreased expression of MHC class I (3). Furthermore, the stage of cellular differentiation of certain tumor cell types has also been inversely correlated with the caste of NK cell cytotoxicity, with poorly differentiated cells showing significantly higher susceptibility to NK cell-mediated lysis than well-differentiated cells, which appear to brandish more sensitivity to chemotherapy-mediated jail cell expiry (38, 39). Given this evidence, Kozlowska et al. proposed a dual approach combining established chemotherapy/radiotherapy regimens (which better target well-differentiated tumor cells), with immunotherapy (which preferentially eliminates CSCs/poorly differentiated tumor cells) (39). Although these researchers recommended this method with oral cancer specifically in heed, it is not difficult to speculate that widespread use of this methodology may be forthcoming.
Conclusion
The theory of NK cell immunotherapy has gained momentum in recent years. This review summarized the most prominent methods past which NK cells are being manipulated for potential therapeutic uses, including population propagation, inhibition of NK cell suppression mechanisms, and enhancement of NK cell target recognition. Numerous studies have shown extensive progress in both managing NK prison cell proliferation and lifespan, every bit well every bit in the directly targeting of tumor cells and tissues. Although much has been discovered, there remains much to larn before such therapies volition become widespread. Further cultivation of specific NK prison cell therapies by both biochemical and clinical studies is necessary before dissemination is possible.
In addition, no firm guidelines exist for the application of NK cell-based therapies in exercise. While evidence to date seems to support its use as a complementary therapy to existing treatment regimens, the potential for an sectional NK jail cell-based polytherapy cannot be ignored.
Finally, more than research must exist completed on the theory of malignancy itself, and on the individual characteristics of specific tumors. Further evaluation of these unique identifiers may help to determine fifty-fifty more precise therapeutic methods, and grade the basis for future tumor staging and treatment guidelines.
Author Contributions
Both authors have made noun contributions to the review of literature, which included drafting the manuscript and revising it critically for important intellectual content. Both have given terminal blessing of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of whatever commercial or fiscal relationships that could be construed as a potential disharmonize of involvement.
Acknowledgments
The authors thank Dr. Jennifer Boardman for her assistance in text editing and revising, Ms. Carmella DiBiasi for her help in figure creation, and Mr. Samuel Clemens for his wit and wisdom.
References
3. Luna JI, Grossenbacher SK, Murphy WJ, Canter RJ. Targeting cancer stalk cells with natural killer jail cell immunotherapy. Expert Opin Biol Ther (2016) 17:313–24. doi:x.1080/14712598.2017.1271874
CrossRef Full Text | Google Scholar
8. Dahlberg CIM, Sarhan D, Chrobok Grand, Duru Ad, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor action. Front Immunol (2015) six:605. doi:ten.3389/fimmu.2015.00605
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Martínez-Lostao L, Anel A, Pardo J. How practise cytotoxic lymphocytes impale cancer cells? Clin Cancer Res (2015) 21(22):5048–56. doi:10.1158/1078-0432.CCR-xv-0685
CrossRef Full Text | Google Scholar
12. Imai K, Matsuyama South, Miyake S, Suga G, Nakachi K. Natural cytotoxic activeness of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet (2000) 356(9244):1795–9. doi:10.1016/S0140-6736(00)03231-1
CrossRef Full Text | Google Scholar
13. Rubnitz JE, Inaba H, Ribeiro RC, Pounds Due south, Rooney B, Bell T, et al. NKAML: a pilot report to make up one's mind the condom and feasibility of haploidentical natural killer prison cell transplantation in babyhood acute myeloid leukemia. J Clin Oncol (2010) 28:955–9. doi:10.1200/JCO.2009.24.4590
PubMed Abstruse | CrossRef Full Text | Google Scholar
15. Vogel B, Tennert K, Full F, Ensser A. Efficient generation of human natural killer cell lines past viral transformation. Leukemia (2014) 28:192–5. doi:ten.1038/leu.2013.188
CrossRef Full Text | Google Scholar
16. Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia past haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion poly peptide. Blood (2014) 123(25):3855–63. doi:10.1182/blood-2013-10-532531
PubMed Abstract | CrossRef Total Text | Google Scholar
17. Wei M, Marino J, Trowell A, Zhang H, Peraino JS, Rajasekera PV, et al. Diphtheria toxin-based recombinant murine IL-two fusion toxin for depleting murine regulatory T cells in vivo. Poly peptide Eng Des Sel (2014) 27(9):289–95. doi:10.1093/protein/gzu034
CrossRef Total Text | Google Scholar
18. Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, et al. Preactivation with IL-12, IL-fifteen, and IL-eighteen induces CD25 and a functional high-affinity IL-two receptor on homo cytokine-induced retentivity-like natural killer cells. Biol Blood Marrow Transplant (2014) twenty(iv):463–73. doi:ten.1016/j.bbmt.2014.01.006
PubMed Abstruse | CrossRef Full Text | Google Scholar
nineteen. Zwirner NW, Ziblat A. Regulation of NK cell activation and effector functions by the IL-12 family of cytokines: the example of IL-27. Front end Immunol (2017) 8:25. doi:10.3389/fimmu.2017.00025
CrossRef Full Text | Google Scholar
20. Issekutz A, Derfalvi B, Käsermann F, Rowter D. Potentiation of cytokine-induced proliferation of human natural killer cells by intravenous immunoglobulin Thousand. Clin Immunol (2015) 161(2):373–83. doi:10.1016/j.clim.2015.08.005
CrossRef Full Text | Google Scholar
22. Watanabe M, Kono K, Kawaguchi Y, Mizukami Y, Mimura K, Maruyama T, et al. NK cell dysfunction with down-regulated CD16 and upwardly-regulated CD56 molecules in patients with esophageal squamous cell carcinoma. Dis Esophagus (2010) 23(8):675–81. doi:x.1111/j.1442-2050.2010.01073.x
PubMed Abstruse | CrossRef Total Text | Google Scholar
23. Petricevic B, Laengle J, Singer J, Sachet K, Fazekas J, Steger One thousand, et al. Trastuzumab mediates antibody-dependent prison cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med (2013) 11:307. doi:10.1186/1479-5876-11-307
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Wang Westward, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front end Immunol (2015) 6:368. doi:10.3389/fimmu.2015.00368
CrossRef Full Text | Google Scholar
25. Zhou Q, Gil-Krzewska A, Peruzzi Yard, Borrego F. Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-neoplasm immunotherapy. Clin Exp Immunol (2013) 173(1):131–9. doi:10.1111/cei.12095
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Li Yard, Xing S, Zhang H, Shang S, Li 10, Ren B, et al. A matrix metalloproteinase inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody immunotherapy in breast cancer by reprogramming the tumor microenvironment. Oncol Rep (2016) 35(3):1329–39. doi:10.3892/or.2016.4547
CrossRef Full Text | Google Scholar
27. Miller MA, Sullivan RJ, Lauffenburger DA. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin Cancer Res (2017) 23(3). doi:x.1158/1078-0432.CCR-16-0869
CrossRef Full Text | Google Scholar
28. Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol (2000) ane(2):119–26. doi:x.1038/77793
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Tan L, Han Due south, Ding Due south, Xiao W, Ding Y, Qian 50, et al. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int J Nanomedicine (2017) 12:3095–107. doi:10.2147/IJN.S128032
PubMed Abstract | CrossRef Full Text | Google Scholar
thirty. Mancusi A, Ruggeri Fifty, Urbani East, Pierini A, Massei MS, Carotti A, et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces nonrelapse mortality. Claret (2015) 125:3173–82. doi:10.1182/blood-2014-09-599993
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Ruggeri 50, Capanni Yard, Casucci K, Volpi I, Tosti A, Perruccio Yard, et al. Office of natural killer alloreactivity in HLA-mismatched hematopoietic stalk cell transplantation. Claret (1999) 94(1):333–9.
Google Scholar
32. Wiernek A, Foley B, Zhang B, Verneris MR, Warlick Eastward, Gleason MK, et al. targeting natural killer cells to acute myeloid leukemia in vitro with a CD16x33 bispecific killer prison cell engager (Bike) and ADAM17 inhibition. Clin Cancer Res (2013) 19(xiv):3844–55. doi:10.1158/1078-0432.CCR-13-0505
CrossRef Full Text | Google Scholar
33. Cooper LJN, Maiti SN. Chimeric antigen receptors on T cells. In: Schwab M, editor. Encyclopedia of Cancer. tertiary ed. Berlin, Frg: Springer Berlin Heidelberg (2011). p. 806–x.
Google Scholar
34. Han J, Chu J, Chan WK, Zhang J, Wang Y, Cohen JB, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII heighten killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep (2015) 5:11483. doi:10.1038/srep11483
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Jewett A, Human Y, Cacalano N, Kos J, Tseng H. Natural killer cells as effectors of pick and differentiation of stem cells: role in resolution of inflammation. J Immunotoxicol (2014) xi(four):297–307. doi:ten.3109/1547691X.2013.877104
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Kozlowska AK, Topchyan P, Kaur K, Tseng HC, Teruel A, Hiraga T, et al. Differentiation by NK cells is a prerequisite for constructive targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer (2017) 8(4):537–54. doi:ten.7150/jca.15989
PubMed Abstruse | CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01061/full
0 Response to "G Dranoff Difference Between Innate and Adaptive Immunity Nature Reviews"
Post a Comment